
Abstracts of the 22
nd
National Congress of Digestive Diseases / Digestive and Liver Disease 48S2 (2016) e67–e231
e177
still unknown. To explore the correlation between gut microbiota
modulation and symptoms improvement in patients undergoing
rifaximn treatment.
Material and methods:
Rifaximin 1200 mg/daily was administered
for 10 days to patients with ulcerative colitis (UC), Crohn’s disease
(CD), irritable bowel syndrome (IBS), diverticular disease (DD) and
hepatic encephalopathy (HE). Inclusion criteria were: no exposure
to antibiotics, pre-/pro-biotics and bowel colonoscopy preparation
for at least one month, and omnivore normocaloric diet for at least
one year. Fecal samples were collected and symptoms were assessed
at baseline and at the end of treatment. Clinical improvement was
evaluated by Mayo score for UC, CDAI for CD, IBS-SSS for IBS, GSS
for DD, and West Haven classification for HE. Fecal microbiota
composition was assessed by a metagenomic gene-targeted
approach (16S rRNA) using the Roche 454 GS Junior ad Qiime
pipeline. Biostatistic analysis was performed using R-statistics
packages.
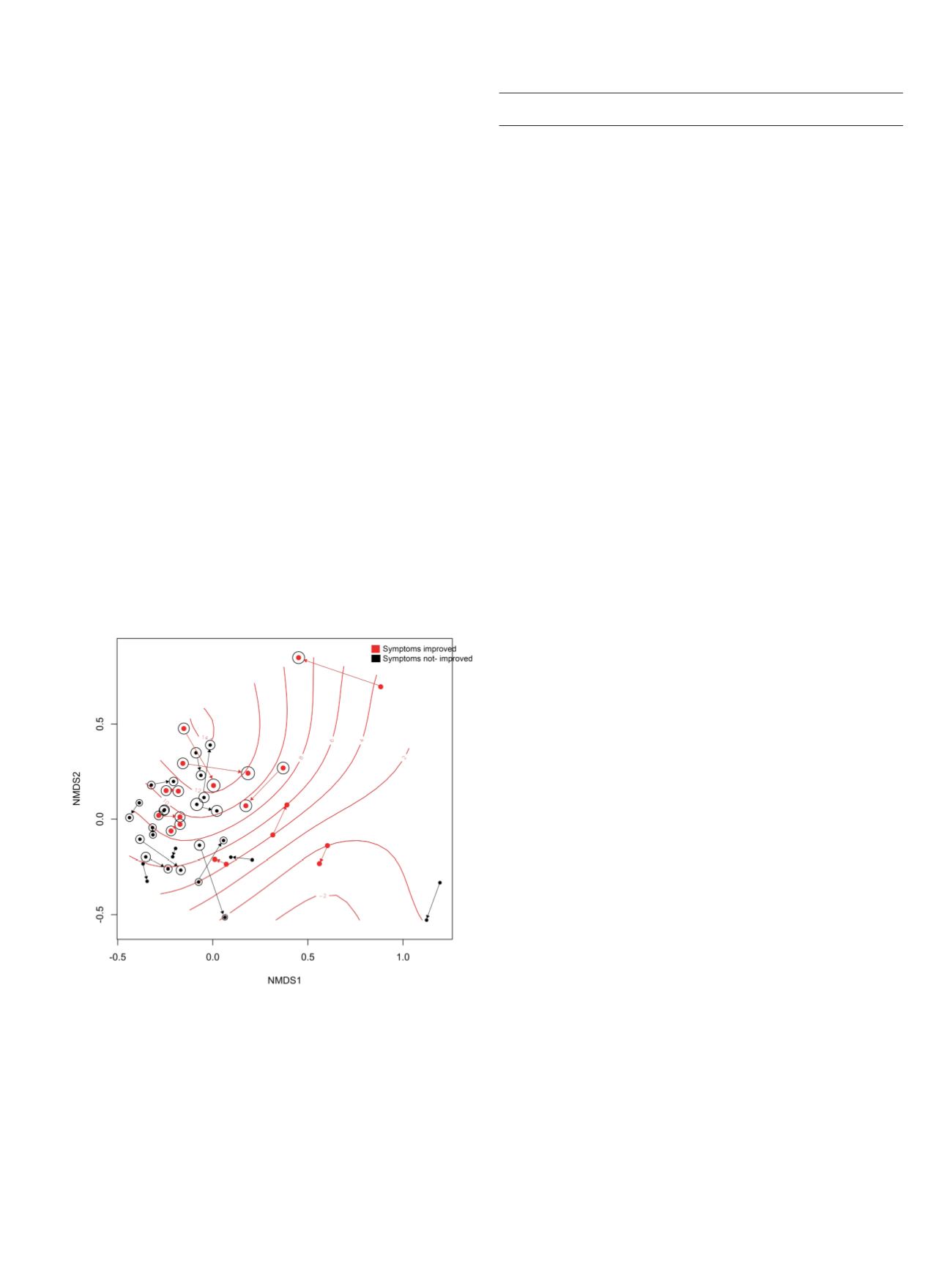
Results:
Twenty-five patients were included in the study. Clinical
improvement was observed in 10 (40%) patients after rifaximin
treatment. Nonmetric multidimensional scaling (NMDS) ordination
on Bray Curtis distance highlighted a significant clustering of
patients who experienced clinical improvement compared to those
who did not (p=0.047; PERMANOVA). Differential abundance
analysis revealed an increased abundance of Faecalbacterium
prausnitzii in case of symptoms amelioration after rifaximin
treatment (improved post vs pre: logFC =1.96; p=0.05; not improved
post vs pre: logFC=-0.37; p=0.810 Figure 1 and 2). The post-treatment
between-groups comparison confirmed a significantly higher
abundance of Faecalbacterium prausnitzii in those patients whose
symptoms improved after rifaximin (logFC =4; p=<0.0001). Clinical
improvement was also paralleled by a significant increase in
bacterial alpha-diversity (p=0.024).
Conclusions:
Beneficial bacteria abundance is increased in patients
with gastrointestinal diseases and hepatic encephalopathy who
achieve clinical improvement after rifaximin treatment. This
mechanism may mediate rifaximin efficacy in different pathologic
settings.
P.10 Liver 2
P.10.1
REACTIVATION OF HEPATITIS B VIRUS IN CANCER PATIENTS
TREATED WITH CHEMOTHERAPY FOR SOLID TUMORS. IS THE
PROPHYLAXIS REALLY REQUIRED?
Federico A.
1
, Dallio M.*
1
, Brancaccio G.
2
, Iodice P.
3
, Fabozzi A.
4
,
Del Prete S.
3
, Ciardiello F.
4
, Gaeta G.B.
2
, Loguercio C.
1
1
Division of Hepatogastroenterology, Second University of Naples,
Naples, Italy,
2
Division of Infectious Diseases, Second University of
Naples, Naples, Italy,
3
Division of Oncology, S. Giovanni di Dio Hospital,
Frattamaggiore (NA), Italy,
4
Division of Oncology, Second University of
Naples, Naples, Italy
Background and aim:
Reactivation of hepatitis B virus (HBV) during
cancer chemotherapy has become an emerging clinical challenge.
High rates of HBV reactivation, 38% to 54%, are now recognized
in HBV-positive patients undergoing hematopoietic stem-cell
transplantation and treatment for hematological malignancy,
especially malignant lymphoma. Less clear is the magnitude of risk
for clinically significant HBV reactivation with chemotherapy for
non-hematological tumors. Aim of this study is to evaluate the risk
of HBV reactivation in carriers and occult carriers of HBV cancer
patients treated with chemotherapy for solid tumors.
Material and methods:
Two hundred sixty-seven patients with
solid tumors were consecutively enrolled, between March 2013 and
February 2014 at two Oncological Division in the Campania Region in
Southern Italy. Before beginning the study, as a screening procedure,
all patients underwent viral marker status (HBsAg/HBsAb, HBcAb,
anti-HCV), liver function test with alanine amonitransferases (ALT),
and liver ultrasonography.
In HBsAg positive patients we evaluated hepatitis B e-antigen/
antibody (HBeAg/HBeAb), HBV-DNA (with real-time fluorescent
PCR).
HBV carriers were followed every 3 months by ALT, HBV DNA; occult
carriers of HBV were followed every 3 months by ALT and HBsAg.
Patients with hypertransaminasemia and HBV-DNA positivity were
treated with tenofovir (245 mg/day).
Results:
Out of the 267 patients, 13 (4.8%) were HBsAg positive, of
whom 6 were documented inactive carrier and 7 had chronic liver
disease (1 compensated cirrhosis). Thirty-two patients (12%) were
HBsAg negative/HBcAb positive and were classified as potential
occult carriers of HBV.
Among patients with HBsAg positive, 12/13 were anti-HBe positive
and 1 patient was HBeAg positive. All patients with chronic liver
disease had an HBV-DNA level >2000 IU/mL and carried genotype D
of HBV. Six occult carrier and one inactive carrier patients were also
anti-HCV positive.
None of the patients undergo therapy with one of the following
drugs: corticosteroids at high dose (>10 mg/day) for long time,
cyclophosphamide, methotrexate. None of the patients had a
reactivation of HBV over 18 months (range 2-24) of observation. The
patient who was HBeAg positive at the enrolment seroconverted
to anti-HBe during the course of treatment with tenofovir after
6 months. The antiviral agents were well tolerated and were
not associated with any unexpected or additional toxicities to
chemotherapy.
Conclusions:
Our study showed that none of the patients presented
an HBV reactivation. Thus, it appears reasonable to avoid HBsAg
and anti-HBc screening in these patients since anti-HBc result is
not relevant to clinical decision. Clearly, screening strategy should
be revised periodically, according to survey results on HBsAg
prevalence in cancer patients.


















