
e78
Abstracts of the 22
nd
National Congress of Digestive Diseases / Digestive and Liver Disease 48S2 (2016) e67–e231
Loss of response (LOR) during the first year of treatment with
ADA is relatively frequent and the main factor associated with
this phenomenon is the reduction of drug serum concentration
to subtherapeutic levels due to the development of neutralizing
antidrug antibodies. As a consequence, dosing ADA trough levels
has been proposed as a useful tool in the therapeutic management
of patients who experience LOR and in the identification of patients
at risk of anti-TNF therapy failure.
Material and methods:
The aim of our prospective study was to
evaluate whether ADA TL a week 8 may predict long term clinical
remission in a single-center cohort of Crohn’s Disease patients. In
order to carry this study out, we included 13 patients with Crohn’s
disease (8 males, median age 41 years, range 21-66) who underwent
ADA therapy and achieved clinical remission after induction. Blood
samples were drawn at standardized time points (0, 2, 8 and 48
week) before ADA administration. ADA TL were measured using
an homogenous mobility shift assay (HMSA; Prometheus Lab, San
Diego, United States). Disease activity was assessed both at week 8
and week 48 by the Harvey-Bradshaw Index (HBI, remission defined
by HBI<5).
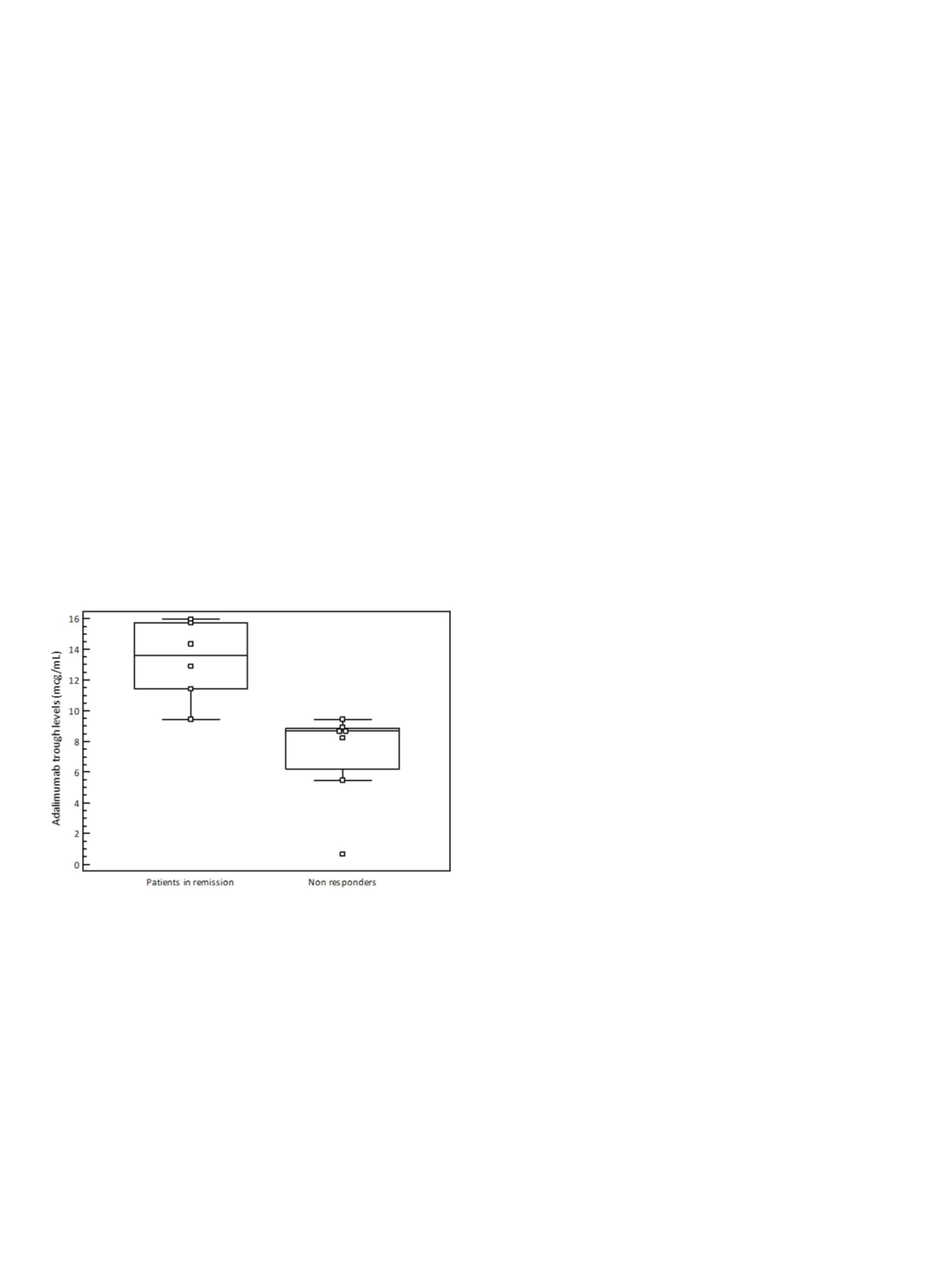
Results:
Among these 13 patients, 7 (53.8%) experienced LOR during
follow-up. We found significantly lower ADA TL at week 8 in patients
who experienced LOR as compared to patients who maintained
remission during the follow-up (8.66 mcg/mL, range 0.68-9.46 mcg/
mL versus 13.63 mcg/mL, range 9.45-15.97 mcg/mL; P=0.0023).
Receiver Operating Characteristic curve identified an ADA TL cut-off
of 8.93 mcg/mL as the threshold with the highest accuracy for
identification of patients who maintained remission (AUROC 0.976,
95% confidence interval 0.715-1; specificity 85.71%, sensitivity
100%).
Conclusions:
Patients who experienced LOR to ADA during long-
term follow-up (48 weeks) have significantly lower ADA TL at week
8 as compared to patients in remission. Furthermore, an ADA TL
concentration cut-off of 8.93 mcg/mL can be used to accurately
identify patients who maintain long term clinical remission. We
suggest that the assessment of ADA TL at week 8 can be used as
a predictive tool for long term clinical response, although these
preliminary results need to be confirmed in larger series.
OC.02.8
A PROSPECTIVE “REAL LIFE” STUDY ON ADALIMUMAB EFFICACY
IN STEROID-DEPENDENT CROHN’S DISEASE PATIENTS: RESULTS
FROM A LONG TERM FOLLOW-UP
Orlando A.*
1
, Renna S.
1
, Cappello M.
2
, Di Mitri R.
3
, Mocciaro F.
3
,
Mazza M.
2
, Giunta M.
4
, Mendolaro M.
2
, Craxì A.
2
, Cottone M.
1
1
DiBiMis, Division of Internal Medicine, “Villa Sofia-Cervello”
Hospital, Palermo University, Palermo, Italy,
2
DiBiMis, Department of
Gastroenterology and Hepatology, Palermo University, Palermo, Italy,
3
Gastroenterology and Endoscopy Unit, ARNAS Civico-Di Cristina-
Benfratelli Hospital, Palermo, Italy,
4
Gastroenterology and Endoscopy
Unit, “Villa Sofia-Cervello” Hospital, Palermo, Italy
Background and aim:
Adalimumab (ADA) is effective in the
induction and maintenance of steroid-free remission in patients
(pts) with steroid-dependent Crohn’s disease (CD). We have already
reported data on efficacy and prognostic factors of response of ADA
(80/40 or 160/80 mg every other week followed by 40 mg every
other week) in 110 steroid-dependent pts. At week 6, 91% of pts
have had a clinical benefit (remission: 45.5%, response: 45.5%). At
the end of the follow-up (mean 14.6 months), 80.9% of responders
have maintained the clinical benefit (remission: 64.5%, response:
16.4%). Only higher induction regimen was related to remission
at week 6. At the end of the follow-up, none of the variables were
associated with remission. Up to now no data are available on long
term efficacy of ADA in the setting of steroid-dependent pts.
Material and methods:
All the 110 pts treated in the previous
study were followed up until April 2015 and the following variables
were evaluated at the end of the follow up: maintenance of clinical
benefit, ADA discontinuation, dose exalation, switch to another
biologic, surgical treatment and side effects.
Results:
At the end of the follow up (mean 74.16 ± 10.3 months) only
5 pts resulted lost during the follow-up. Concerning the remaining
105 pts, 42 pts (40%) obtained the clinical benefit: 1) 37/42 (88%)
were still in maintaining treatment with ADA at the dosage of 40
mg sc (of these pts 13/37 [35%] received a weekly maintaining
treatment); 2) 5/37 (12%) discontinued ADA due to mucosal healing.
Sixty-three pts (60%) discontinued ADA: 1) 50/63 (79%) for lost of
clinical benefit (20 of these 50 pts were operated on [40%]); 2) 6/63
(10%) for side effects; 3) 5/63 (8%) for severe endoscopic activity
despite clinical response; 4) 2/63 (3%) died for reason non related
to ADA treatment. Among pts who discontinued ADA 24/63 (38%)
were then effectively switched to another biologic (infliximab or
golimumab). At univariable analysis we did not find variables related
to the treatment outcomes. ADA was well tolerated. Only one pts
developed an acute leukaemia after 2 years of ADA discontinuation.
Conclusions:
This long term“real life” prospective study showed that
ADA is a good maintaining treatment in steroid dependent CD but
1/3 of them needed dose escalation to maintain clinical benefit. The
rate of long term side effects that needed treatment discontinuation
is quite low. In pts intolerant to or with lost of response to ADA a
switch to another biologic is an effective opportunity.
OC.02.9
CROSS-SECTIONAL EVALUATION OF TRANSMURAL HEALING
IN PATIENTS WITH CROHN’S DISEASE ON MAINTENANCE
TREATMENT WITH BIOLOGICS
Rispo A.*
1
, Mainenti P.
2
, Testa A.
1
, Imperatore N.
1
, De Palma G.D.
3
,
Rea M.
1
, Maione F.
3
, Nardone O.M.
1
, Taranto M.L.
1
, Castiglione F.
1
1
Gastroenterology “Federico II” University, Naples, Italy,
2
Radiology
“Federico II” University, Naples, Italy,
3
Surgery “Federico II” University,
Naples, Italy
Background and aim:
Transmural healing (TH) of Crohn’s disease
(CD is a new underexplored and interesting outcome of the concept


















