
Abstracts of the 22
nd
National Congress of Digestive Diseases / Digestive and Liver Disease 48S2 (2016) e67–e231
e149
reported the hydropic degeneration of basal layer, subepithelial T
cell infiltrate, epithelial hyperplasia, hyperkeratosis, acanthosis,
necrotic keratinocytes. The picture was compatible with the
diagnosis of lichen planus. A second anamnesis revealed the
presence of a long lasting oral lichen planus nearby a mobile dental
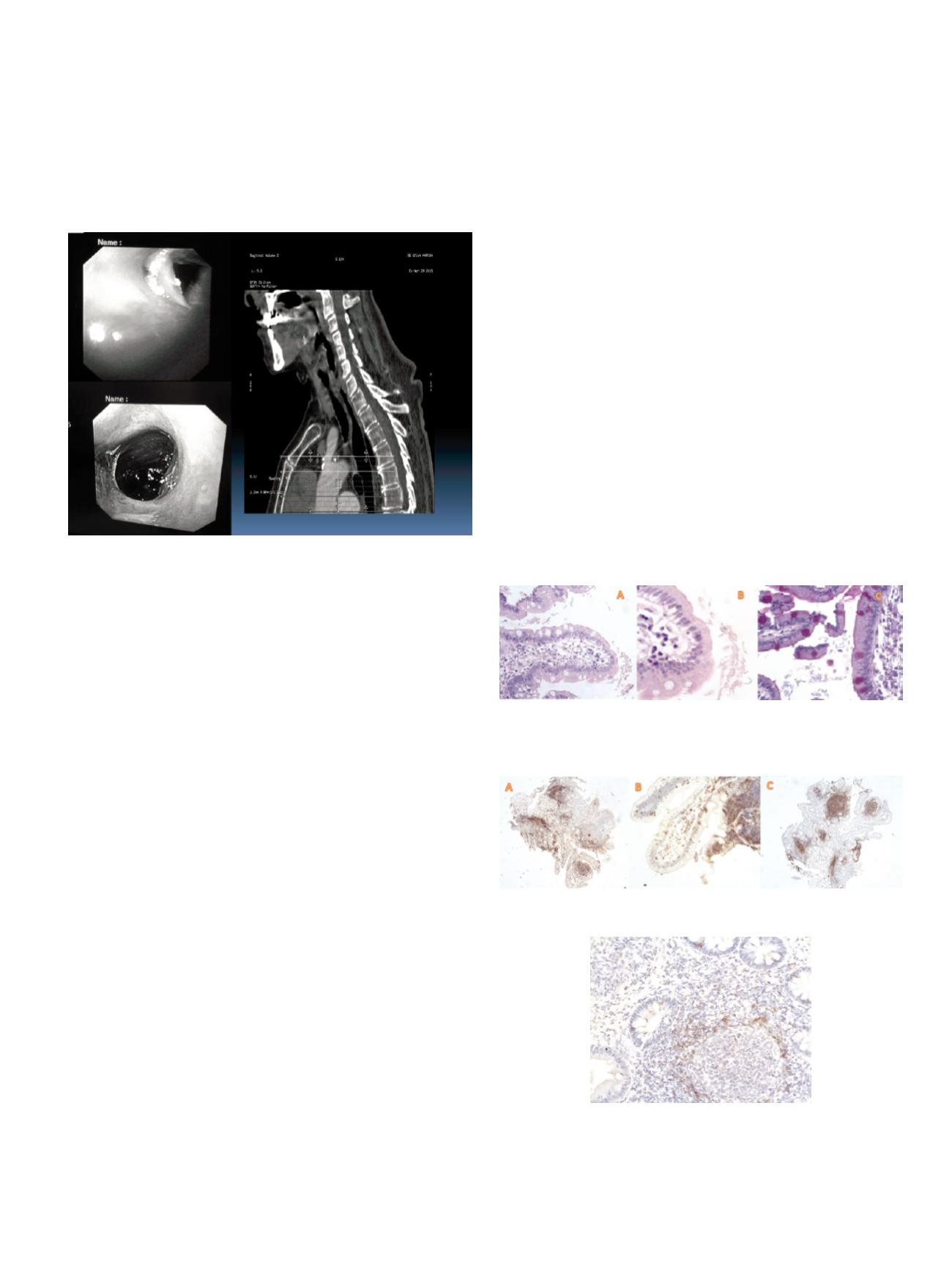
bridge. Patient was treated with endoscopic dilatations by the use of
bougies over a Savary guidewire by increasing the diameters until 9
French (fig 3).
Results:
Patient reported an immediate symptoms relief. Dilatation
in lichen planus stenosis is not the first choice due to the risk of
Koebner phenomenon but this patient the comorbidity did not
allowed systemic steroid therapy.
Conclusions:
Lichen planus is an idiopathic disorder with clinical
manifestation in the skin, mucous membranes, genitalia, hair
and nails. Esophageal involvement by lichen planus is rare,, more
commonly found in middle-aged woman, mostly associated with
oral lesions. It can be missed because a long time (years) is necessary
from the disease onset and the development of dysphagia. Even if
esophageal localization of lichen is rare, it must be considered in
the differential diagnosis of dysphagia and esophageal stricture
especially in elderly women, along with peptic disease and
esophageal adenocarcinoma.
Our case demonstrated that clinical, endoscopic and histologic data
may narrow a broad list of differential diagnoses for the esophageal
stricture and in this case allowed the diagnosis of the rare esophageal
lichen planus.
P.05.6
A RARE CASE OF CELIAC DISEASE IN A PATIENT WITH COMMON
VARIABLE IMMUNODEFICIENCY: WHEN VILLOUS ATROPHY IS
NOT ENOUGH
Morreale G.C.*
1
, Sinagra E.
2
, Rizzo A.
3
, Amvriosadis G.
1
,
Montalbano L.M.
1
1
(1) Gastroenterology Unit, Ospedali Riuniti Villa Sofia-Cervello,
Palermo, Palermo, Italy,
2
(2) Fondazione Istituto S. Raffaele G. Giglio,
Gastroenterlogy and Endoscopy Unit, Cefalù, Cefalù, Italy,
3
(3)
Pathology Unit, Ospedali Riuniti Villa Sofia-Cervello, Palermo, Palermo,
Italy
Background and aim:
We present a case of a woman with a
diagnosis of celiac disease (CD) histological documented by villous
atrophy; other test demonstrated the presence of common variable
immunodeficiency (CVID) complicated by Giardia Lamblia infection,
an other cause of villous atrophy. In this case, HLA and histological
response to a glutenfree diet (GFD) confirmed CD and GFD resolved
clinical manifestation and villous atrophy.
Material and methods:
A 40 years old woman presented with
chronic diarrhea, weight loss of about 8 kg in 2 months, and iron
deficiency anemia (Hb:8 g/dl); in 2013 patient receive diagnosis of
CD based on histological characteristics (marked chronic inflam
mation with intraepithelial lymphocytosis, severe flattening of the
villi “March 3 C”). She started gluten free diet. For persistence of
clinical symptoms despite GFD, an other planned disease revaluation
was performed in 2014. Research of abnormal hemoglobin chains
showed no tassemia and other hemoglobinopathies; laboratory test
showed negative celiac markers (transglutaminase IgA and IgG
antibodies) but very low levels of all classes of immunoglobulin (Ig)
(IgA <6.67 mg / dl, IgG <119 mg / dl, IgM <4.17 mg / dl. Abdomen
ultrasound showed only mild splenomegaly (Dt 13 cm). The
esofagogastroduodenoscopy (EGDS) showed on duodenum mucosa,
nodules of various sizes, up to 5 mm. At Hystological exam: severe
villous atrophy, nodular lymphoid infiltration of the lamina propria,
no evidence of plasma cells, presence of many spheroidal
morphology forms compatible with Giardia lamblia (GI). (Fig 1A,B,C);
CD5 glycoprotein expression positive (as a marker of intraepithelial
T lymphocytes); CD20 negative (B-lymphocytes absent) (Fig. 2A,B,C);
switch on D-Immunoglobulin (IgD) for absence of other classes of
Immunoglobulin in CVID (Fig 3). There was also follicular lymphoid
hyperplasia. CVID complicated by GI infection was diagnosed;
patient started antibiotic therapy with metronidazole; CD was
excluded so it was placed indication to practice free diet. Patient had
an increase of weight but diarrhea and anemia not resolved. A new
research of GI in the stool was negative.
Results:
The patient was referred to our Gastroenterology Unit.
We decided to perform genetic test with evidence of HLA-DQ2
Fig. 1.
(A,B) Hystological view of duodenitis by Giardia Lamblia: many spheroidal
morphology forms CPAS positive stanining (x40 magnification). (C) the trophozoites
of Giardia Lamblia. This parasitic infection is typical of patients with CVID and is
associated with villous atrophy.
Fig. 2.
(A,B,C) CD5 glycoprotein expression positive (as a marker of intraepithelial T
lymphocytes); CD20 glycoprotein are negative(B-lymphocytes absent).
Fig. 3
The switch on D-Immunoglobulin (IgD) for absence of other classes of
Immunoglobulin in CVID.


















