
Abstracts of the 22
nd
National Congress of Digestive Diseases / Digestive and Liver Disease 48S2 (2016) e67–e231
e213
P.17.5
FIBRIN SEALANT (EVICEL®): ENDOSCOPIC INTERVENTIONAL
MANAGEMENT OF BLEEDING GASTROINTESTINAL LESIONS:
PROSPECTIVE, SINGLE-ARM, PILOT STUDY
Staiano T.*
3
, Martinotti M.
1
, Rispo A.
2
, Buffoli F.
4
1
S.C. Chirurgia Generale A.O. Istituti Ospitalieri di Cremona, Cremona,
Italy,
2
DAI Gastroenterologia, Endocrinologia, Chirurgia A.O.U. Federico
II, Napoli, Italy,
3
S.C. Endoscopia Diagnostica e Chirurgia Endoscopica
Fondazione IRCCS Istituto Nazionale dei Tumori, Milano, Italy,
4
S.C.
Endoscopia Digestiva e Gastroenterologia A.O. Istituti Ospitalieri di
Cremona, Cremona, Italy
Background and aim:
The mortality and morbidity rates for
gastrintestinal bleeding lesions are higher in patients with spurting
bleeding, oozing, or a non bleeding visible vessel on endoscopy. The
prognosis of patients presenting with major peptic ulcer bleeding
is improved by endoscopic injection therapy. A high success rate of
70 – 100% has been reported using various agents, such as ethanol,
polidocanol, cyanoacrylates. Evicel
®
is a fibrin sealant consisting of
two components, human clottable fibrinogen and human thrombin.
It is indicated as supportive treatment in patients undergoing
surgery when control of bleeding by standard surgical techniques is
ineffective. It is a new formulation of the previously available fibrin
sealant. Evicel is easy to use and don’t contain synthetic or bovine
aprotinin, reducing potential for hypersensitivity reactions. The aim
of this study was to evaluate the clinical outcomes of patients with
GI bleeding lesions treated with Evicel.
Material and methods:
Between September 2014 and August 2015,
a total of 10 patients with major hemorrhagic lesions (Non Variceal
Upper and Lower GastroIntestinal Bleeding Lesion (NV-ULGIBL))
with active bleeding or a nonbleeding visible vessel were enrolled.
The pts were well matched for age, sex, initial hemoglobin values,
ulcer size and location, and bleeding stigmata.
7 of 10 patients were female (median age 82.9 y). Patients
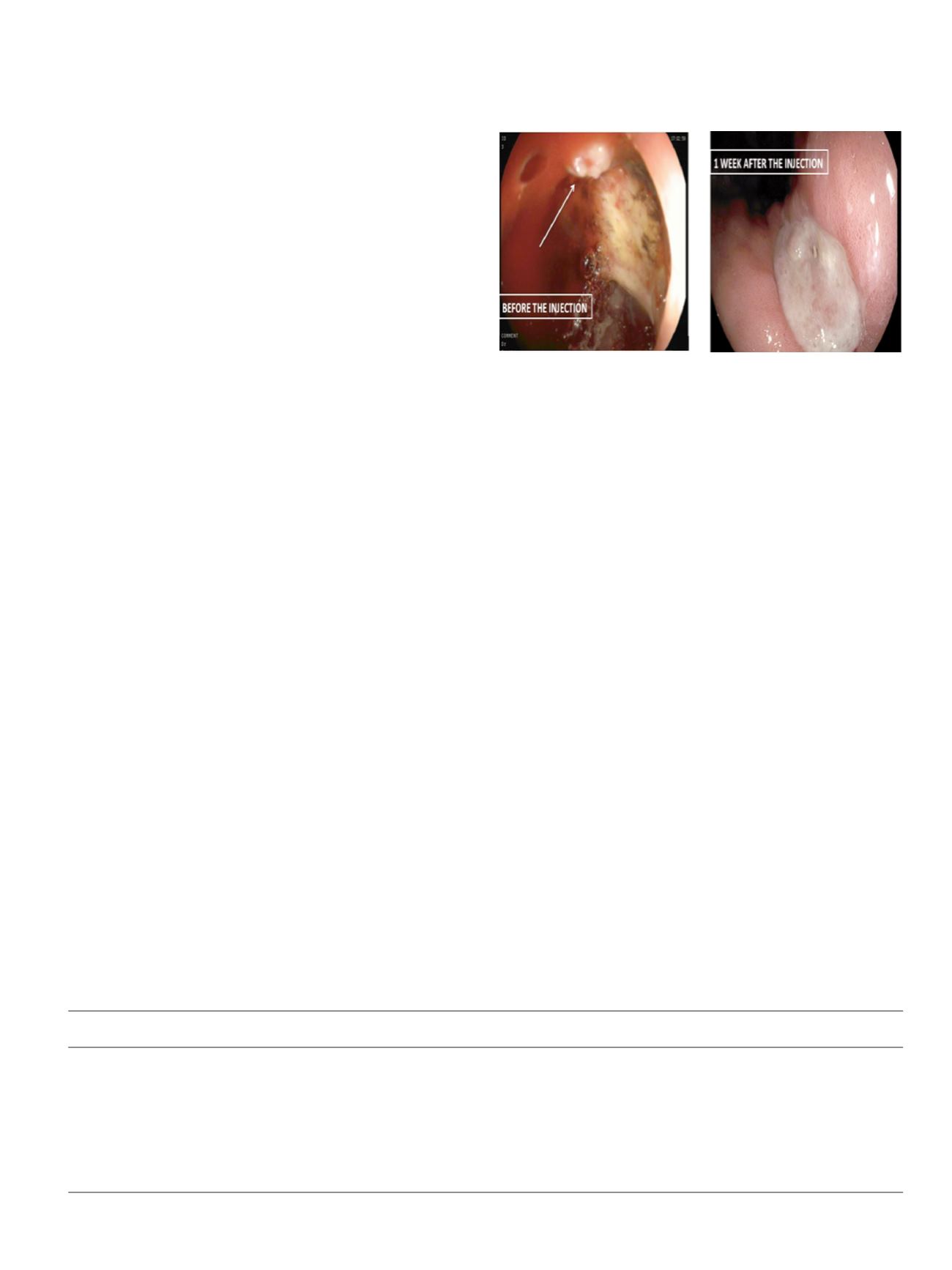
who underwent Evicel injection the fibrin sealant was injected
submucosally at the bleeding site. We opted for the sequential
single lumen injection technique to facilitate injection and diffusion
of the diluted fibrin into the submucosa followed by the thrombin
activator to maximize hemostasis within the target area and avoid
early superficial clot formation. Patients, lesions characteristics and
outcomes are summarized in Table 1 (Fig 1).
Results:
Initial hemostasis was achieved in all cases (100%).
Rebleeding occurs in one case (duodenal ulcer with active arterial
bleeding) and was treated with selective transarterial embolization.
No early or late rebleeding occurred during the follow-up. No
complications or instrument lesions related to Evicel injection were
recorded.
Conclusions:
In our series, fibrin sealant injection was found to be a
potentially safe and effective endoscopic haemostatic treatment
modalities in controlling bleeding from NV – UL GIBL with major
hemorragic stigmata. Due to the the small number of patients and
the absence of randomisation, in our study, no definitive conclusions
could be drawn concerning the use of the Evicel in the treatment of
severe NV ULGIBL bleeding.
P.17.6
QUALITY OF LIFE AFTER GASTRIC BANDING AND GASTRIC BY-
PASS FOR MORBID OBESITY: A PROSPECTIVE COMPARISON
Marchesi F.
1
, Forlini C.*
1
, De Sario G.
1
, Tartamella F.
1
, De Lorenzis G.
3
,
Generali I.
4
, Ricco’ M.
2
, Dall’Aglio E.
5
, De Panfilis C.
4
1
Azienda Ospedaliero Universitaria di Parma, Parma, Italy,
2
Azienda
Provinciale per i Servizi Sanitari della Provincia Autonoma di Trento,
Trento, Italy,
3
Casa di Cura Città di Parma, Parma, Italy,
4
Dipartimento
di Neuroscienze, Università di Parma, Parma, Italy,
5
Unità Operativa
di Diabetologia e Malattie Dismetaboliche, Dipartimento di Medicina
Interna e Scienze biomediche, Azienda Ospedaliero Universitaria di
Parma, Parma, Italy
Background and aim:
Among bariatric surgery evaluation criteria,
improvement in quality of life (QoL) surely represents the most
important. However, the lack of reliable evaluation models has
significantly limited the research in this field. The aim of this study
is to prospectively compare QoL after Roux-en-Y gastric bypass
(RYGB) and laparoscopic adjustable gastric banding (LAGB).
Material and methods:
35 patients submitted to RYGB at “Parma
Hospital” and 35 patients submitted to LAGB at the “Clinica Città
di Parma” were enrolled in the study. Patients were prospectively
submitted to one preoperative and 4 postoperative (1, 3, 6 and 12
months) clinical evaluations including two psychodiagnostic tests:
the Short Form 36 (SF-36) testing the quality of life and the Bariatric
Surgery Satisfaction Questionnaire (BSSQ) assessing the satisfaction
for the intervention. At the same times, comorbidity (i.e glucidic
tolerance, degenerative joint disease etc.) and biometric changes (i.e
weight, height and BMI), have been evaluated. The two groups have
been compared relatively to change in BMI and comorbidities and
the outcome of the two questionnaires.
Results:
Both interventions produced a significant amelioration of
biometric data, significantly higher for RYGB (p=0.05). While at early
controls no significant difference in SF-36 was detected among the
Table 1
(abstract P.17.5)
Characteristics of patients and outcome
Patient
Hemorragic
Successful
Additional
(case)
Sex
Age
Site of bleeding
Stigmata
Hemostasis
Treatments
Rebleeding
Outcome
1
F
89
J-jejunal anastomosis
Ia
Yes
No
No
Favorable
2
F
87
Duodenal ulcer
Ib
Yes
No
No
Favorable
3
F
72
Duodenal ulcer
Ib
Yes
No
No
Favorable
4
M
87
Gastric ulcer (fundus)
Iia
Yes
No
No
Favorable
5
F
78
Duodenal ulcer
Ia
Yes
Yes: clip
Yes: Tae
Favorable
6
F
85
Rectal ulcer
Spurting vessel
Yes
No
No
Favorable
7
F
82
Gastric ulcer (antrum)
Iia
Yes
No
No
Favorable
8
M
37
Gastric ulcer (body)
Ia
Yes
No
No
Favorable
9
M
68
Rectal cancer
Oozing bleeding cancer floor area
Yes
No
No
Favorable
10
F
66
Colo–anal anastomosis
Spurting vessel
Yes
No
No
Favorable


















